usp class vi compliant
All wetted materials are fully biocompatible USP class VI and of animal-free origin or compliant with EMA41001. Optimized clinical workflows Existing workflows make it easy for clinicians to follow policies and benefit from quick access to the latest evidence-based guidance.
Measuring range up to 45 m 148 ft Process connection starting 34 thread or flange Temperature range.

. Smooth interior for excellent flow and performance. Ultem Properties and Material Options. Quick and easy EHR integration Seamlessly access patient data across systems.
The materials used are traceable back to their production batches. Intermittent discharge of cell culture. USP Class VI USP 661 or ISO 10993 for plastic materials.
Components compliant with physiochemical tests. Custom sizes available along with FDA compliant USP Class VI compliant and medical grade options. Pharma and Medical Grade O-rings USP Class VI Seals ISO 10993 Seals Food Grade O-rings FDA Rubber Sheet NSF-51 Seals 3-A Sanitary Rubber Drinking Water O-rings NSF-61 Compliant Gaskets WRAS Compliant Gaskets KTW Compliant Gaskets Good Wear Resistance Good Compression Set Resistance Moderate Short-Term Resilience.
There are also different testing protocols within ISO 10993 and USP Class VI. Low permeability as compared to silicone. Division offers a wide range of polymers and additives to the medical device and industrial sectors with FDA and USP Class VI approvals to assist customers in the development and manufacturer of medical.
MULTIVAC provide a comprehensive range of GMP-compliant packaging automation solutions for the medical and pharmaceutical industries. 21 CFR 820 Compliant. In addition our SCGM is proved to be highly efficient for the expansion and cultivation of human natural killer cells NK cells and human cytokine induced killer cells CIK cells.
Because neither USP Class VI nor ISO 10993 are synonymous with biocompatibility testing asking for a biocompatible rubber can lead to confusion. Food Contact Status compliant. All Drainage to Building Regs Part H 2002.
116-136 as timely if it is made no later than January 4 2021 which is the first business day after January 1 2021. Supply and lay on 100mm C75 concrete bed and haunch Class B polymer concrete linear drain cw Class C galvanised grating and fixtures to levels and falls. Secondary Support Processes.
ISO 10993 and Medical Molding. Additionally Simriz 486 is FDA compliant and USP class VI compliant making it the perfect match for any Food Beverage and Pharmaceutical application. We have many special compounds that can meet the specification you are looking for.
Location_on258 Goddard Rd Lewiston Maine 04240 Tel. 10m and store spoil on site for re-use. And has achieved numerous certifications.
Its unique patented material structure provides an outstanding long-term. By Industry A-K Aerospace. All of our electronic grade adhesives and materials are RoHS and REACH compliant and many are halogen free.
Fluoroelastomers Market worth USD 252 billion by 2028. It is suitable for sterilization by E-beam EtO and Gamma tested to 25 kGy. Utem 1000 Unfilled or unreinforced 1000 series plastics are translucent amber in color and combine exceptional mechanical thermal and electrical properties.
- 100M Business Profiles - 20M Contacts in Asia - 95 data accuracy ----- CCPA and GDPR Aligned Our data is compliant with GDPR and USA privacy laws. Excellent low absorption and adsorption characteristics as compared to silicone. REACH RoHS and California Proposition 65 compliant.
In fact USP Class VI is sometimes seen as a minimum requirement for biocompatibility. Let us know if you require materials to meet the following standards. Meets European Pharmacopoeia 3221 standards.
English 315 KB PDF. Center feed for low-shear operation. WRAS Water Regulations Advisory Scheme Approved Material USP Class VI FDA and EC 19352004 EU 102011 compliant TA-luft VDI Guideline 2440 ABS-PDA Pamphlet 95 the chlorine institute DNV-GL.
196 to 450 C 321 to 842 F Pressure range. Need help choosing an o-ring material. This is an optional feature you can study W3Schools without using My Learning.
Tapflo pumps have become an important element of equipment for installations especially those used for transporting hazardous liquids. Some medical device applications require adhesives formulated to pass biocompatibility testing such as USP Class VI or ISO 10993. Track your progress with the free My Learning program here at W3Schools.
We also offer certifiable compounds that meet AMS 3216G USP class VI FDA compliant Dupont Company U2A and SU2A sheet specification. Advanced data enrichment Makes data more actionable seamlessly using data mapping and. With SoleSource you can expect.
FDA listed and USP Class VI compliant. Log into your account and start earning points. USP Class VI Compliant.
The flow paths are produced under controlled conditions and packed in a clean room environment class ISO 7 using validated protocols. CellGenix GMP SCGM is an optimized xeno-free medium used for the serum-free expansion of low numbers of isolated human hematopoietic stem and progenitor cells HSCsCD34 cells. Eastar 6763 is a clear amorphous material that can be molded and extruded with ease.
Our solutions are safe and environmentally friendly and meet the high quality requirements imposed by standards and directives. Include connection to SW system. Simriz 498 - Outstanding Performance Simriz 498 is the ultimate FFKM material.
Met requirements for Plastic Class VI 121C 250. 1 to 400 bar 145 to 5 800 psi. Flexelene MFX Tubing This medical-grade tubing is soft pliable nonallergenic noncytotoxic and ultra-low in extractables.
21 CFR 820 Compliant. This notice provides that the IRS will treat a contribution to a single-employer defined benefit pension plan with an extended due date of January 1 2021 pursuant to 3608a1 of the Coronavirus Aid Relief and Economic Security Act CARES Act Pub. Secondary Support Processes.
Eastar 6763 copolyester meets ISO 10993 andor USP Class VI biocompatibility requirement. We offer a full range of products that are USP Class VI certified or ISO 10993 approved. Excavate for connecting pipework to depth ne.
UL MIL AMS ASTM Food Grade FDA 21 CFR 1772600 compliant Medical Grades FDA USP Class VI or ISO 10993. FMP54 - premium device for high-temperature and high-pressure applications mainly in liquids. Meets USP Class VI requirements.
By Industry A-K Aerospace. Meets various ISO and USP standards including Class VI. And has passed the API 6FA fire test.
USP Class VI Compliant. Its excellent performance properties include clarity toughness good melt strength no dusting no stress whitening good heat. We only crawl and index publicly available email addresses and phone numbers such as those that are accessible from websites and social media.
Concentric tubes to introduce feed and remove supernatant.

Parker V1274 75 Usp Class Vi Biocompatibility O Ring United Seal
Dursan Passes Usp Class Vi Testing Why Is That Important

Double Bagged Usp Class Vi Liquid Funnels

Standard Fda And Usp Class Vi Compliant Materials For All Types Of Hygienic Connections Repassa

Rulon 641 Usp Approved And Fda Compliant Tristar Plastics
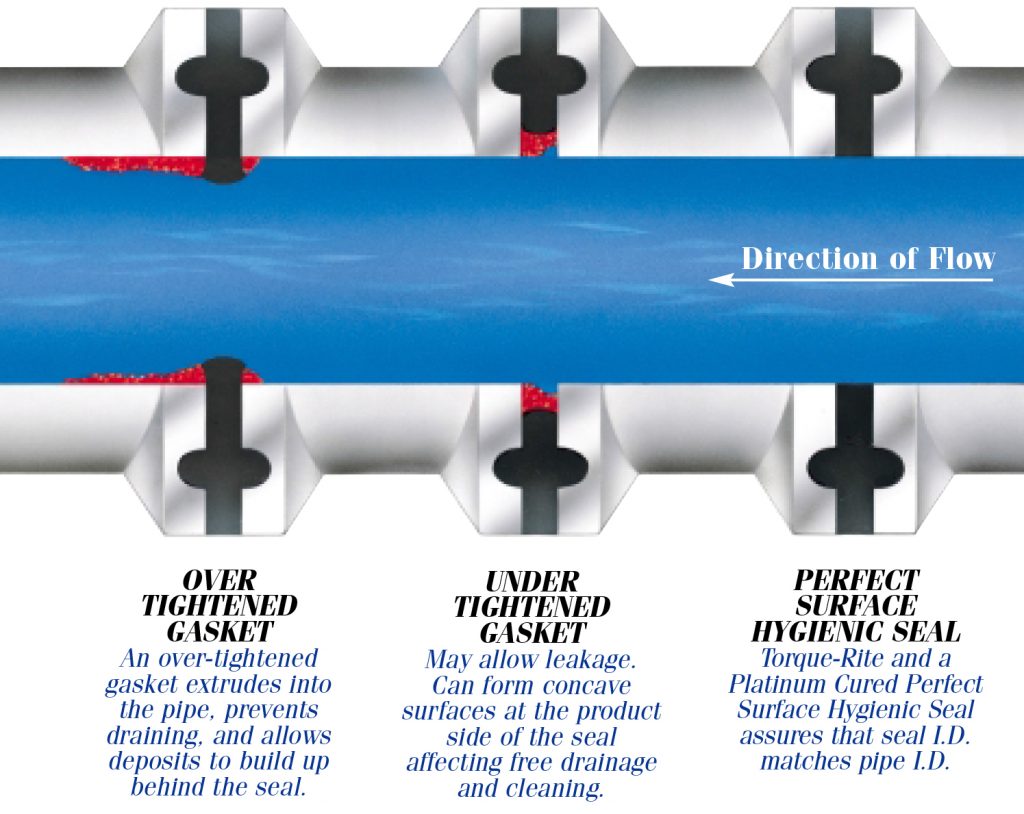
Standard Fda And Usp Class Vi Compliant Materials For All Types Of Hygienic Connections Repassa

Usp Class Vi Foster Corporation

Iso 10993 Vs Usp Class Vi Medical Molding And Bicompatible Rubber The Rubber Group

Usp Class Vi Testing Aft Fluorotec

Fda Usda Nsf51 Usp Class Vi Compliant Seals Products

Usp Class Vi What Is It And How Does It Apply To Elastomers Barnwell

Why You Need Certified Usp Class Vi Silicones Specialty Silicone Products Inc





